Detergents contain surfactants or surface active agents. Surfactants are molecules that are made up of a head that is hydrophilic (water loving) and a tail that is hydropobic (loves grease and dirt). In the wash, the fecal matter and urine attach to the hydrophobic section of the surfactant and are lifted away with the water by the hydrophilic section. Once they are lifted away with the water, they washed away because they have no receptors left to attach back to fabric.
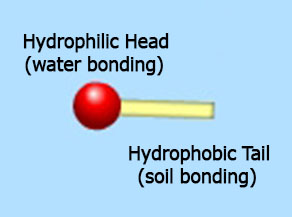
As mentioned above, the surfactants job is to attach to soil on one end and water on the other, thus pulling soil OUT of fabric and away through the drain holes attached to the water.
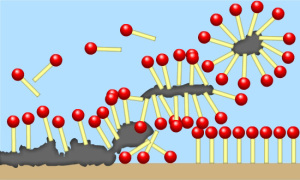
Diagram of how soil is attached and suspended in water via surfactant molecules. (Source)
Surfactants cause a reduction in surface tension of the water. “Surface tension is a force present within the surface layer of a liquid that causes the layer to behave as an elastic sheet. It is the force that supports insects that walk on water, for example. Surface tension is caused by the attraction between the molecules of the liquid.” (Source)
Suds or bubbles or “foam is created when the surface tension of water is reduced and air is mixed in, causing bubble formulation.” “When water sprays from a tap in a small basin, you can see bubbles form, but they burst very soon. This is due to the fact that the surface tension of the normal water is high and it tends to draw the water molecules into the main body of the water, to the point where the thickness of the bubble wall is too thin to remain intact and quickly bursts. Instead, the surface tension of the soapy water is much lower: about a third of the pure water, and so the molecules of the bubble are less stressed and it can last longer.” (Source) In simple terms, surfactants reduce the surface tension of the water, which allows bubbles to hang around on the the surface of the water easily. It doesn’t mean there is excess detergent or that all of the detergent is not being removed, it only means it’s easier for bubbles to form and for them to stay intact.
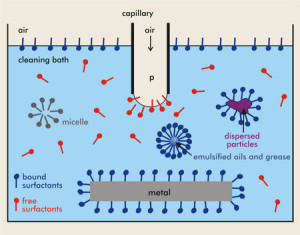
Diagram of surfactants attaching to soil and suspending them in water. Free surfactants are also suspended in water. Agitation and reduction in surface tension create bubbles/suds to form on the surface. (Source)
Softened water allows the formation of bubbles/suds more readily, so having soft water or the addition of a water softener such as Borax or Calgon to your cycles will automatically increase the amount of suds present. The same is true of bleach, it is a mild water softener. In the event of a hard water strip (done with water softeners), followed by a bleach soak (mild water softener), there is a high likelihood of having an increased amount of suds present in the following wash. Softeners being released into the water via the laundry causes this increase.
In a laundry setting, in a TL washing machine, the majority of bubbles/suds are sitting on the surface of the water and are quite literally thrown out during the spin. The reason clothes don’t FEEL sudsy or slimy or soapy even after one rinse is because the suds are not embedded in the fabric, they are removed during the force of the spin and are mostly made up of air. Peeking in your washer during the rinse cycle and seeing suds is no reason to panic, seeing them at that point is NO indication you need additional rinses or that you used too much detergent. During the rinse cycle, any remaining surfactants will attach to the water and be removed as it goes down the drain, BUT during the rinse cycle, some surfactant is still present and therefore surface tension is still reduced so there is still a high propensity for bubbles. There is no need to look in your washer during the cycle, it’s unnecessary and causes unnecessary worry. If your diapers and other laundry don’t feel soapy or slimy when FINISHED, everything is FINE.
There are a couple of instances where suds CAN be a problem. However, they have nothing to do with too much detergent or needing extra rinses and everything to do with what is called a suds cushion. Two things can cause this, using a detergent that is NOT hE formulated or UNDERloading your hE FL washer.
Detergents which are NOT hE formulated:

UNDERloading your HE FL washer:
Too few clothes in a washer can cause an increase in the potential of a suds cushion. In this case, there is not enough fabric moving around to break up and pop the suds produced through friction/tumbling. The suds continue to grow as the cycle progresses and the cushion does not allow proper friction with the sides of the machine and disbursement of soil into the water from the laundry, which results in less than clean laundry. Most hE machines clean most efficiently between ⅔-¾ capacity.
REFERENCES:
Before we begin…
Throughout this document, I will be discussing the ingredients and methods for our DIY Mix strip. Although we have found stripping with Mighty Bubbles to be as effective as this method, and stripping with RLR to also work, those products have proprietary formulae that the companies will not disclose. If you’re stripping with either one of those products, rest assured that the chemical reactions and other mechanics of stripping are very similar, but not quite exactly what you’ll read about here.
What goes into a DIY mineral strip?
CALGON
- Chemical name: sodium hexametaphosphate
- Formula: Na6P6O18
- pH: 8 (slightly alkaline)
- Calgon is a name-brand product based in the UK. It is sold around the world as a limescale preventative and laundry additive, though it’s sometimes a bit harder to track down in stores. Regardless of where you are, you should be able to order Calgon online.
- Calgon is the only water softener in our DIY mix that is non-precipitating, meaning minerals which are dissolved in the water remain dissolved when they are bound.
BORAX
- Chemical name: Sodium borate (sometimes called sodium tetraborate or disodium tetraborate)
- Formula: Na2B4O7·10H2O
- Solubility: 175-180 grams per liter
- pH: 9.5 (somewhat alkaline)
- Borax is precipitating, meaning that when it binds to minerals, they are taken out of the water to form solid particles that must be physically rinsed away.
- Reacts with calcium and magnesium through the following reactions:
- Ca2+ (aq) + Na2B4O7 (aq) → CaB4O7 (s)↓ + 2 Na+ (aq)
- Mg2+ (aq) + Na2B4O7 (aq) → MgB4O7 (s)↓ + 2 Na+ (aq)
WASHING SODA
- Chemical name: sodium carbonate
- Also known as soda crystals or soda ash
- Formula: Na2CO3
- Solubility: 200-300 grams per liter
- pH: 11 (very alkaline)
- Washing soda is precipitating, meaning that when it binds to minerals, they are taken out of the water to form solid particles that must be physically rinsed away.
- Reacts with calcium and magnesium through the following reactions:
- Ca2+ (aq) + Na2CO3 (aq) → CaCO3 (s)↓ + 2 Na+ (aq)
- Mg2+ (aq) + Na2CO3 (aq) → MgCO3 (s)↓ + 2 Na+ (aq)
DETERGENT
HOT WATER
- We recommend soaking at the hottest possible temperature your water will go. Most home water heaters are set around 120-140 degrees, so we can reasonably assume your strip water will start out around 115 degrees Fahrenheit.
TIME
- We recommend soaking until the water is cool, or about 4 hours.
How does stripping work?
Stripping diapers works through three main mechanisms:
- a chemical reaction between the water softeners and minerals present in the water
- heat
- pH
CHEMICAL REACTIONS
The main way the ingredients in our DIY mix remove buildup from diapers is through a chemical reaction. The chemical reactions for borax and washing soda are very similar – in fact, the reactions are identical except one uses boron and the other uses carbon. Both water softeners bind to calcium and magnesium in the water, causing the calcium and magnesium to solidify (or precipitate) so they can be rinsed away without getting trapped in the fibers of your diapers.
HEAT
Soaking the diapers in hot water helps in two ways:
- The hot water helps dissolve or loosen any non-mineral buildup, such as soap scum (from using homemade or a detergent with lots of sodium cocoate) or soil and bacteria (from diapers not getting adequately clean before they are re-soiled).
- The hot water also opens up the pores and fibers in the diapers, allowing the strip water to penetrate deeper into the multiple thick layers of cloth.
PH
The strip soak water is fairly high pH, and using such an alkaline solution helps loosen and dissolve non-mineral buildup such as soap scum or trapped soil and bacteria.
Why are all these ingredients necessary?
- Calgon is necessary to the mineral strip because, as the only non-precipitating water softener in our mix, it most thoroughly removes minerals from the diapers. When borax and washing soda react to form precipitated calcium and magnesium, the precipitated minerals (teeny tiny solid particles) are susceptible to being trapped in the fibers of your diapers. This will only happen if there is some other agent involved that can trap these particles – such as soap scum or inadequately cleaned filth. Calgon, however, as a non-precipitating water softener, carries no risk of any particles getting trapped, regardless of other factors.
- Please note that trapping the precipitated minerals is not a concern during a normal wash routine. This is because during a proper wash routine, your diapers will not have any soil or soap scum that can trap the particulate matter.
- Borax and washing soda react with calcium and magnesium in very similar ways, but there are some key differences in the two chemicals:
- Borax reacts more easily than washing soda. The molecular bonds between borate and sodium are weaker than the molecular bonds between carbon and sodium. It takes less energy to break the bonds of a borax molecule, which makes it a more efficient water softener than washing soda.
- Washing soda is more soluble than borax, meaning more washing soda than borax will dissolve in the same amount of water. Although we don’t get close to the saturation point with either borax or washing soda during a DIY strip, it’s definitely easier to dissolve the washing soda.
- Washing soda has a much higher pH than borax. Washing soda’s pH is around 11; borax’s pH is around 9.5. Creating a more caustic or alkaline solution is helpful in loosening or dissolving any waste or soap scum that might be clinging to diaper fibers.
- Adding detergent to the DIY strip mix helps in two ways. First, adding something containing surfactants works in the strip just like it does in the wash – the surfactants bind to soils with one end and to water with the other, allowing them to lift away the icky things trapped in your diapers. Second, we strongly recommend using a detergent with enzymes for stripping. The enzymes will help break down protein-based soils, such as those made by human waste.
When you strip your diapers, the following things happen:
- The hot, alkaline water opens up the fibers of the fabric and looses any soil or scum.
- The strip water penetrates deep into the fabric of the diapers, where the water softeners can react with minerals that have become trapped.
- The surfactants and enzymes in the detergent help break down, bind, and lift away soils.
- When you stir or agitate the stripping diapers, the movement helps the surfactants lift away soils and allows new “pockets” of buildup to become exposed so they can be thoroughly cleaned.
What about stripping when there’s no mineral buildup?
Sometimes, we will recommend a strip even when there is no mineral buildup. These cases are
- When diapers have been washed repeatedly in something that will prevent mineral buildup, but not get them clean, such as:
- When “cloth safe” detergent has been used for an extended period of time
- When homemade “detergent” (without soap) has been used for an extended period of time
- When a clearly inadequate amount of conventional detergent has been used for an extended period of time
- When diapers have been washed repeatedly in something that will build up and coat fibers, such as:
- When liquid All® detergent has been used for an extended period of time
- When a soap has been used to clean diapers, such as detergents made of saponified coconut oil, for an extended period of time
- When homemade “detergent” (with soap) has been used for an extended period of time
- When soap nuts or soap berries have been used for an extended period of time
- When diapers have been washed with synthetic fabric softener for an extended period of time (one accidental wash with Snuggles is OK! Many washes over several months are not!)
- When Charlie’s Soap or another dangerous detergent has been used
When we recommend stripping without mineral buildup, the process is exactly the same. However, in these cases, the surfactants, enzymes, heat, and alkalinity play a much larger role in getting diapers clean. If you need to strip because of a non-mineral issue, we recommend you strip using Grovia Mighty Bubbles. This method employs surfactants and enzymes at a much higher concentration than our DIY mix, which is really intended for mineral strips.
What stripping will NOT do
- The chemicals used in a DIY mineral strip will not react with the polyurethane laminate of your diapers.
- Stripping does not disinfect or sanitize your diapers
- Stripping will not solve wash routine issues – although it is an effective and generally safe (for the diapers) treatment, stripping diapers is hard on them. Extended soaking of any kind will reduce the life of your diapers, which is also why we do not recommend a wet pail. In order to keep stripping from being a regular thing, you must know how to properly clean your cloth diapers.
- Stripping will not remove petroleum- or zinc-based stains, such as those from using a “non-cloth-safe” diaper cream. It may help lift some of the petroleum away, but generally speaking, scrubbing those stains with a toothbrush and degreaser (such as dish washing liquid) and then rinsing thoroughly before proceeding with a normal wash routine is a far better course of action.
- Stripping is not an effective stain removal tool. It may help, and diapers will probably look brighter and fresher when stripping is finished, but generally speaking, stripping should not be done to solve staining problems.
OMG! STRIP ALL THE THINGS!
Hold on there, Thunder. While stripping diapers is a wonderful tool to help rejuvenate diapers, it is not a gentle process. Extended soaking of diapers is hard on fabric, especially when soaking in such a caustic solution. That’s why we don’t recommend stripping diapers regularly or even frequently. Stripping is not the way you get clean diapers. You get clean diapers with a good wash routine. Stripping is an extreme process used to correct months or years of improper washing, not something you do just for fun. If your diapers are clean out of the wash, there is no need to strip.
One rare outcome of stripping is leaking diapers. Although our survey data reported that 95% of survey respondents experienced no leaking or only temporary leaking, that does not by any means discount the other 5%. As we work to determine the exact cause of the leaks, you should know:
- the vast majority of the time, any leaks are temporary and resolved with additional washing with a good wash routine; proper agitation, enough detergent for a heavily soiled load, the proper water level, and using liners or cloth-safe diaper cream are all important to resolving leak issues.
- PUL is water resistant, not water proof. With a strong enough stream of water focused on one spot in the PUL, any diaper will experience leaks, regardless of stripping.
- recently stripped inserts will absorb liquids much more readily than those caked with mineral buildup, coated with scum, or containing unwashed soil. They are also more prone to releasing those liquids more quickly in the form of compression leaks. If you have been double-stuffing diapers prior to stripping, try moving to a single insert to reduce the incidence of compression leaks.
- all normal leaking guidelines apply. Do the diapers fit properly? Is there enough absorbency (are the inserts fully saturated when the leaks occur)? Is it pointed down? Have non-cloth-safe creams been used?
References
There is a lot of concern from parents about chemicals and ingredients we expose our children to, especially when cleaning cloth diapers. This stems from the thought that detergent chemicals are 1) harmful to baby in any type of exposure and 2) they embed themselves into the cloth. We are here to help you understand why these two things do not happen when using a real detergent.
While eating/ swallowing or inhaling laundry products is toxic (please always keep any cleaning product WAY out of the reach of your children), washing fabric with a detergent is how it is designed.
DETERGENTS ARE MADE UP OF:
- Surfactants: molecules that are made up of a head that is hydrophilic (water loving) and a tail that is hydropobic (loves grease and dirt). In the wash, the fecal matter and urine attach to the hydrophobic section of the surfactant and are lifted away with the water by the hydrophilic section. Once they are lifted away with the water, they washed away because they have no receptors left to attach back to fabric.
- Builders: The amount of builders varies depending on your detergent. A builder is much like a booster (Oxyclean, Borax, Calgon) as its job is to soften the water and remove minerals like calcium from binding to the clothes. They allow the Surfactants to bind to fecal matter and urine instead of to the minerals. They are safe for both your diapers and your baby.
- Bleaches: There are many different kinds of bleaches, including sodium hypochlorite (NaClO) or liquid bleach, hydrogen peroxide (H2O2), peracids and bleach activators. A bleach activator such as the popular Tetraacetylethylenediamine (TAED) are needed for hydrogen peroxide to work at temperatures below 104* F or 40* C. These are safe for your diapers and your baby.
- Colorants: These are considered safe in detergent. What is left in the water is treated in a water treatment plant and outside high concentrations is considered safe for aquatic life.
- Optical Brighteners: These are used to make fabrics look whiter by reflecting back more blue light thus reducing the yellow appearance. While for users with very sensitive skin optical brighteners can be an irritant, for most, there is no concern during use.
ADDITIONAL INGREDIENTS:
- Enzymes: Not necessary for detergent, but improves performance. Enzymes are specifically chosen to attach and lift off protein stains. In the case of cloth diapers, the enzyme helps to break down the fecal matter and urine. Since they occur naturally in our environments, they are eco-friendly and will not hurt baby, plants or other animals.
- Fragrance: this varies between brands. Those with extremely sensitive skin should avoid fragrances. Some mainstream companies such as P&G use only biodegradable perfumes/fragrances.
REFERENCES:
Cloth Diaper Manufacturers/Sellers than recommend Real Detergent:
Proper Washing of Fabrics:
Bleach for Disinfecting Safely:
Why Certain Alternate Methods of Disinfection are NOT effective (Vinegar, TTO, etc)
The Difference Between Soap and Detergent:
Hard Water:
Water pH:
Grapefruit Seed Extract/GSE:
Vinegar:
Tea Tree Oil/TTO:
Using the Sun to disinfect/UV:
Phenols:
Oil of Oregano:
Oxygen Bleach:
General Sanitizing and disinfecting:
Prepping:
Bamboo/Rayon:
Charcoal Bamboo:
“OxyVin”- dangerous stripping method combining OxyClean and Vinegar *NOT ADVISED*:
Borax:
Sodium Metasilicate/Why We Do NOT Recommend Charlie’s Soap:
Urine pH:
PUL:
Tide:
“China Cheapies” diaper Sunbaby Reviewed 2 Years Later:
Do Not use DAWN in a Washing Machine:




